TNMU Professor Becomes an Expert of Group 10B of the European Pharmacopoeia
Professor Liliia Lohoida, Head of the Department of Pharmaceutical Chemistry at Ternopil National Medical University, has been elected as an expert of Group 10B of the European Pharmacopoeia, which is responsible for the development, verification, and revision of pharmacopoeial monographs.

The European Pharmacopoeia is a key regulatory document of the Council of Europe that establishes mandatory standards of quality, safety, and efficacy for medicinal products, pharmaceutical substances, and excipients for its member states. Its requirements are applied by regulatory authorities and manufacturers to ensure a high level of public health protection.
Leading experts from across Europe are involved in the work of the European Pharmacopoeia. They participate in the preparation, updating, verification, and harmonization of monographs, as well as in the development of modern analytical methods for quality control of pharmaceutical products.
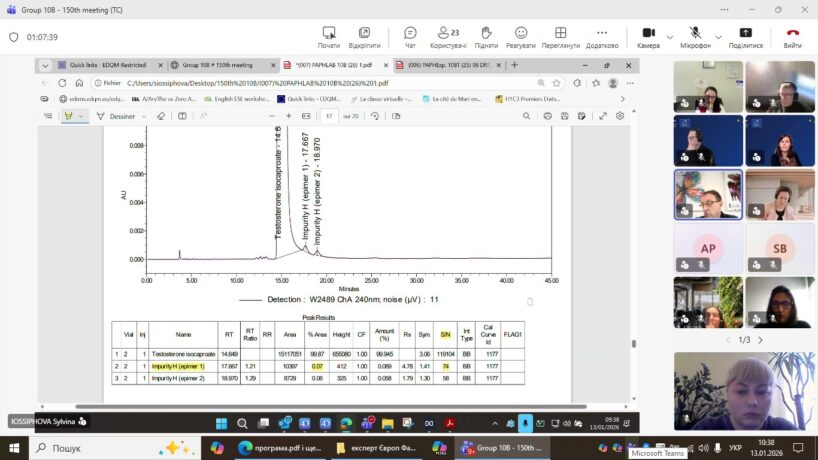
On 13–14 January 2026, a regular meeting of Expert Group 10B was held, during which 63 monographs were discussed. Some of the monographs were verified (reproduced) by experts under laboratory conditions. The next meeting of the expert group is scheduled for 8–9 April 2026 and will be held online. An in-person meeting of the experts is planned for 15–16 September 2026 in Strasbourg.
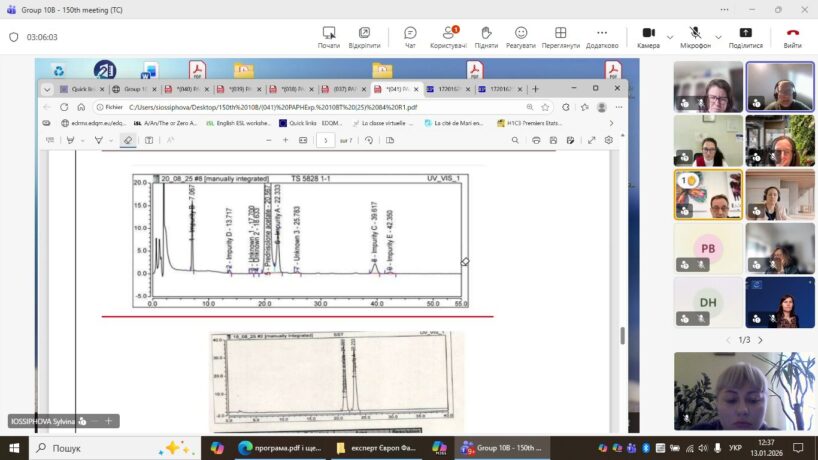
The election of a Ukrainian representative to Expert Group 10B of the European Pharmacopoeia is an important step toward strengthening Ukraine’s participation in shaping European standards for medicinal product quality and integrating the national pharmaceutical system into the European regulatory framework.
